

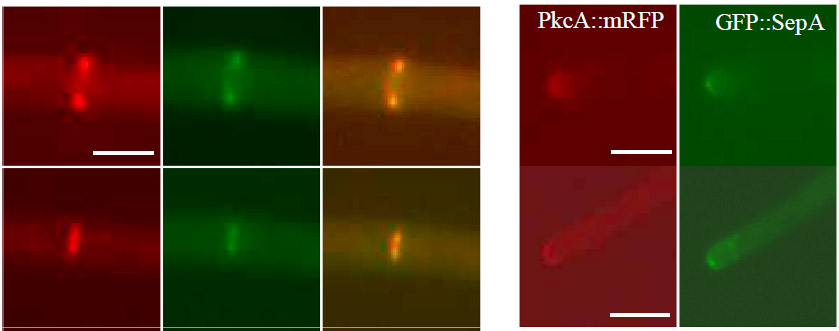
The image below demonstrates colocalization of SepA (green) and PkcA (red) at sites of septation (left-hand panels) and the growing hyphal apex (right-hand panels).
 |
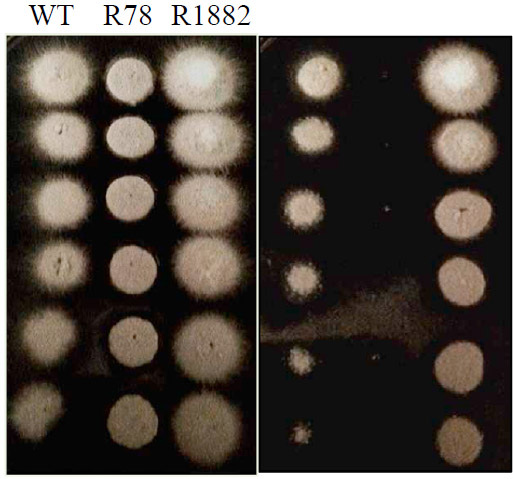
We also demonstrate a functional relationship between the two protein, in that elevated expression of SepA compensates for deficiency in the function of PkcA. The image below shows the inability of a strain bearing a PkcA-mutation (R78) to grow in the presence of the wall-inhibitory chemical Calcofluor White (CFW), while the wild type strain (A28) and a strain containing the PkcA mutation plus over-expression of SepA (R1882) can tolerate the inhibitory chemical. The left-hand panel shows all three strains growing in the absence of CFW, and the right-hand panels show growth in the presence of CFW.
 |
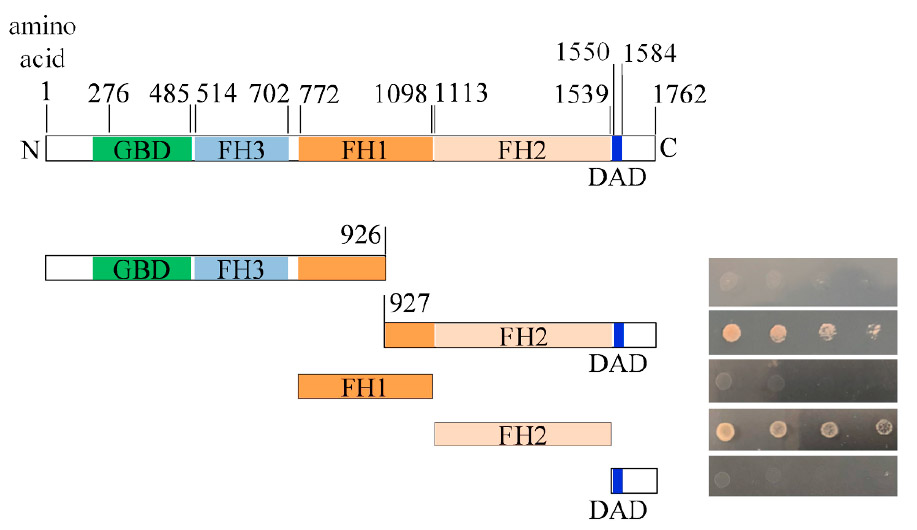
To investigate whether the colocalizing proteins are actually physically binding to each other, we have employed the Yeast-Two-Hybrid procedure along with protein truncation (selective removal of protein domains). In the image below, the upper diagram depicts the various domains of SepA. Below that are shown four different truncation constructs that were paired with full-length PkcA in the Y2H procedure. Protein-protein interaction between PkcA and SepA is shown by the ability of yeast colonies to grow in the absence of the amino acid histidine, a property shown only by the two constructs having intact FH2 domains.
 |
Literature:
Jackson-Hayes, L., Z. Atiq, B. Betton, W. T. Freyaldenhoven, L. Myers, E. Olsen, and T. W. Hill. 2019. "Aspergillus nidulans protein kinase C forms a complex with the formin SepA that is involved in apical growth and septation." Fungal Genetics & Biology 122: 21-32.

