

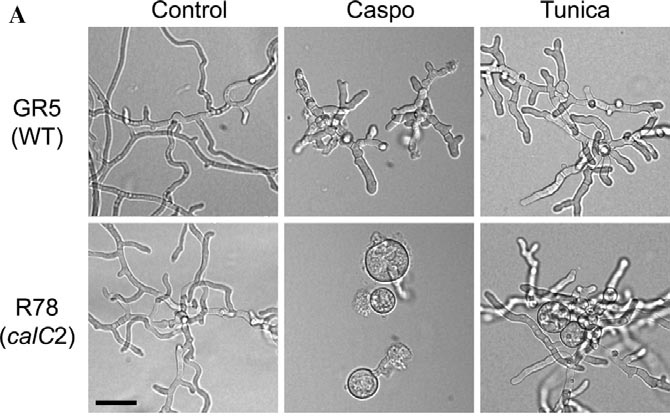
We have demonstrated that the genetic defect in the Aspergillus nidulans calC2 mutation is a point mutation in the A. nidulans orthologue of PKC, designated PkcA. The mutation affects the C1B domain, causing hypersensitivity to Calcofluor White along with other drug sensitivities that indicate a defect in cell wall integrity (Teepe et al., 2007). This suggests strong parallels to the Cell Wall Integrity signalling pathway of yeasts, in which PKC functions as a central player.
 |
|
One of several examples of the calC2 mutant's elevated sensitivity to wall compromising agents is the excessive swelling of spores during germination in the presence of Caspofungin (inhibitor of glucan synthesis) and Tunicamycin (inhibitor of glycosylation). | |
|---|---|
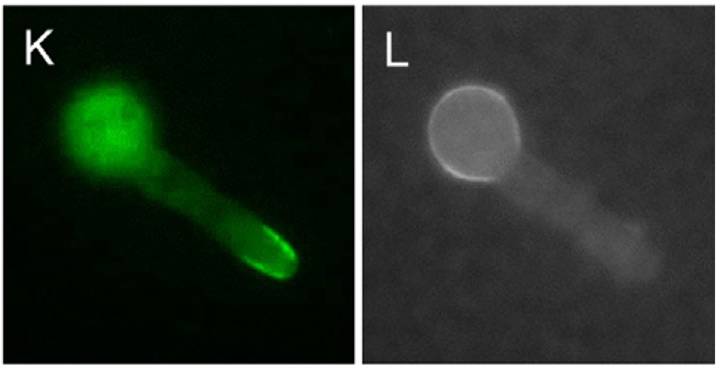
A PkcA::GFP chimera localizes to hyphal apices and growing septa, as well as to the conidiogenous apices of phialides, indicating a role for PkcA in polarized cell wall growth. These observations support the hypothesis that the role of PkcA in A. nidulans, is comparable to that played by Pkc1p in the Saccharomyces cerevisiae cell wall integrity pathway.
 |
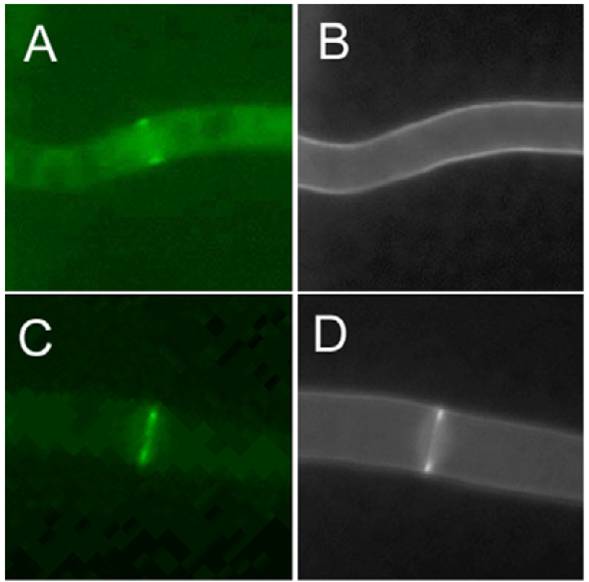 |
In each image pair, the color image shows localization of PkcA::GFP and the grayscale image the localization of chitin (stained with CFW). The first pair illustrates PkcA targeting to hyphal apices - the remaining two pairs show dynamic localization to forming septa. | |
|---|---|
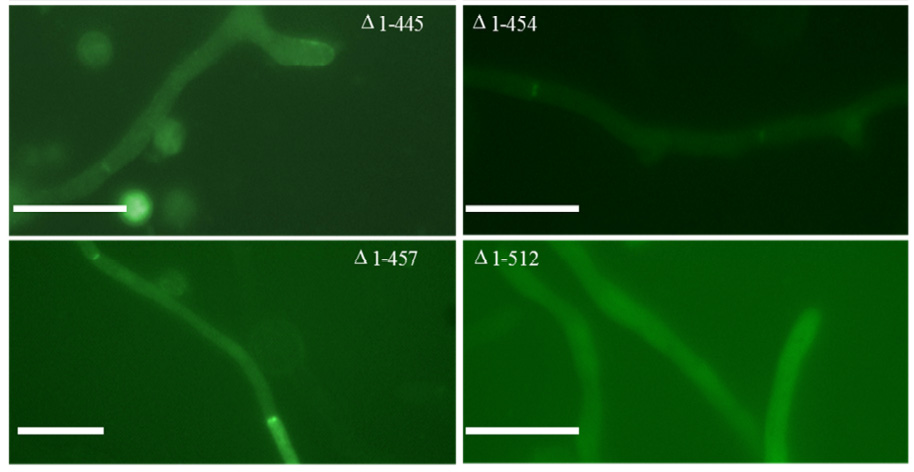
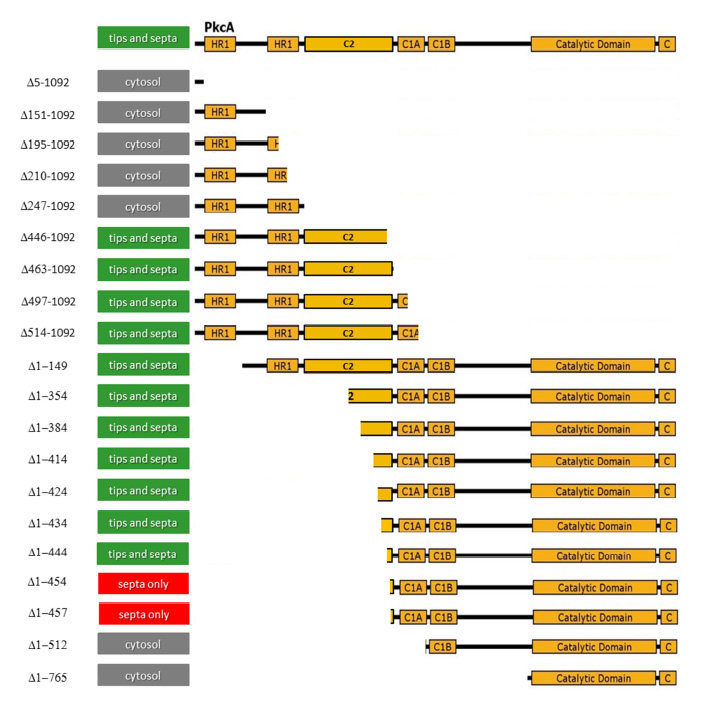
Recent work (Jackson-Hayes et al., 2015) has identified two specific regions of PkcA that are involved in targeting the protein to tips and/or septa . Truncated versions of PkcA were produced carrying either N-terminal or C-terminal GFP tags, and monitored for ability to localize to tips or septa (see below).
 |
|
Analysis of the localization pattern identifies two specific regions that must be present in the PKC molecule in order to localize to one site or another.
 |
|
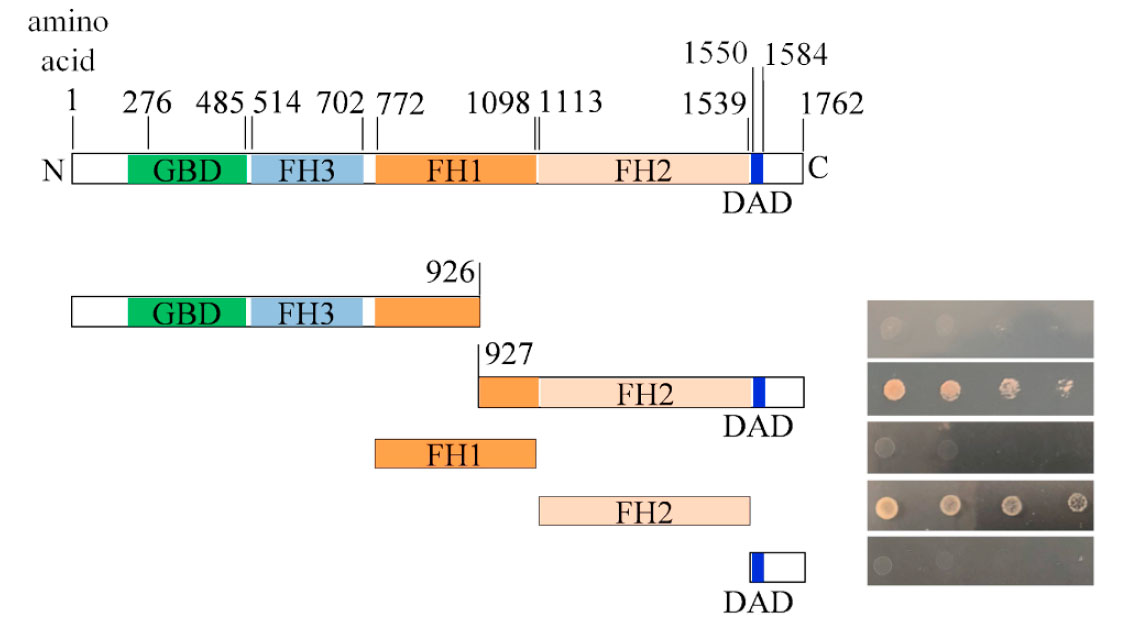
More recent work (Jackson-Hayes et al., 2019) with Yeast-Two-Hybrid (Y2H) and Bimolecular Fluorescence Complementation (BiFC) demonstrates that PKC and the formin SepA form a complex at growth sites (apices and forming septa), which is dependent upon the FH2 domain of SepA.
 |
|
Literature:
Teepe, A. G., D. M. Loprete, Z.-M. He, T. A. Hoggard, and T. W. Hill. 2007. "The protein kinase C orthologue PkcA plays a role in cell wall integrity and polarized growth in Aspergillus nidulans". Fungal Genetics & Biology 44: 554-562.
Jackson-Hayes, L., T. W. Hill, D. M. Loprete, C. DelBove, J. Shapiro, J. Henley, and O. Dawodu. 2015. "Two amino acid sequences direct Aspergillus nidulans protein kinase C (PkcA) localization to hyphal apices and septation sites." Mycologia 107: 452-459.
Jackson-Hayes, L., Z. Atiq, B. Betton, W. T. Freyaldenhoven, L. Myers, E. Olsen, and T. W. Hill. 2019. "Aspergillus nidulans protein kinase C forms a complex with the formin SepA that is involved in apical growth and septation." Fungal Genetics & Biology 122: 21-32.

